Naming Chemical Compounds
- STEM To Go

- Sep 11, 2020
- 5 min read
Updated: Jul 22, 2021
In chemistry, you may notice that different rules apply to different types of compounds, including those that determine their systematic names. If you're having trouble deciding when to use which rule, continue reading to get some information that will help you distinguish between the different compounds based on their chemical formula, as well as how you would go about naming them!

To start off, you must be able to identify what type of compound you're trying to name. The different types of compounds that you will most likely encounter are ionic compounds, acids, hydrates, and binary molecular compounds.
Before getting into the different types, you should also know how to identify whether an element is a metal or a nonmetal. The majority of the elements are metals, and they are generally on the left side of the periodic table, while nonmetals are on the right side. The official line which divides them is known as the "staircase", which are the elements boron, silicon, germanium, arsenic, antimony, and tellurium — also identified as the metalloids. Referring to the color-coded periodic table below, the elements in the staircase are boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), and tellurium (Te), which are in a green-gold color. The elements on the left side of the staircase are metals (excluding H - hydrogen which is in bright green along with other gases), and have a positive charge, while the elements on the right are nonmetals, and have a negative charge.

Now that you have a good understanding of the types of elements, let's get into the different types of compounds!
Ionic Compounds (Metal-Nonmetal)
Ionic compounds are those that contain metals (cation - positive charge) and nonmetals (anion - negative charge). These compounds have a neutral charge since the positive and negative charges will cancel out and end up as zero when you calculate the total charge. You can remember that they are called ionic compounds since they are formed by ions, which are atoms that aren't neutral, and have a +/- charge.
In addition, the anion can also be a polyatomic ion, meaning a compound formed by two nonmetals, which still makes it a nonmetal with a negative charge.
To name these compounds, you would use the rule:

For example, the systematic name of table salt (NaCl) would be sodium chloride.
Na is the chemical symbol for sodium, Cl is the chemical for chlorine, so you would take the root "chlor-" and add "-ide" to get "chloride". As a result, its full name would be "sodium chloride"
Remember that this formula is essential, as it is the base that can be applied to multiple formulas because ionic compounds can also be combined with other elements and compounds to form even more compounds.
Acids (H+-Nonmetal)
Acids are the easiest to identify, as they are compounds with hydrogen in the beginning. However, naming them is a little complicated, as the rule, you apply depends on what the cation is. Keep in mind that all of them end with "acid", but you have to remember which rule to apply for the first part of the name.
If the anion is a nonmetal element, so it ends with "-ide" (ex: fluoride, oxide, nitride, carbide, etc.), you would use the formula:
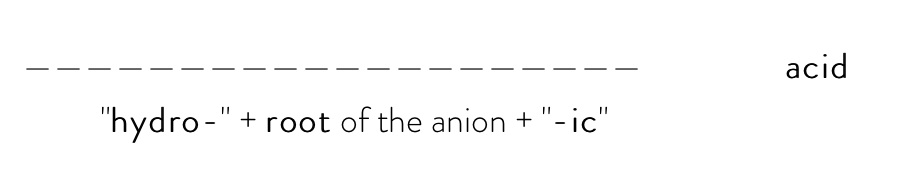
For example, the systematic name of H3P would be hydrophosphoric acid.
P is the chemical symbol for phosphorus, so you would take root "phosphor-" and add "hydro-" in the beginning and "-ic" at the end to get "hydrophosphoric". Then, add acid to the end of the final name to get "hydrophosphoric acid."
If the anion is a polyatomic anion or compound formed by two nonmetals, it depends on what the name of the polyatomic is, or specifically, what it ends with.
If the polyatomic anion ends with "-ate" (ex: chlorate, sulfate, acetate, carbonate, etc.), you would use the formula:
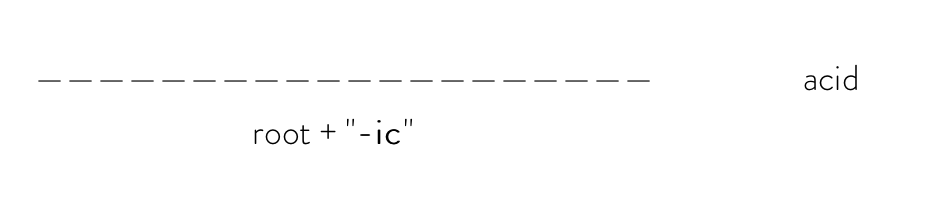
For example, the systematic name of H2CO3 would be carbonic acid.
CO3 is a polyatomic anion called carbonate, so you would take the root "carbon-" and add "-ic" at the end to get "carbonic". Then, add acid to the end of the final name to get "carbonic acid".
If the polyatomic anion ends with "-ite" (ex: nitrite, sulfite, chlorite, etc.), you would use the formula:
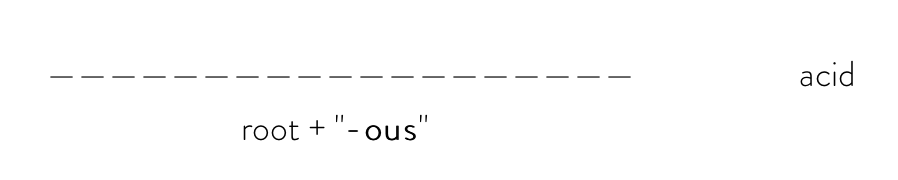
For example, the systematic name of HClO2 would be chlorous acid.
ClO2 is a polyatomic anion called chlorite, so you would take the root "chlor-" and add "-ous" at the end to get "chlorous." Then, add acid to the end of the final name to get "chlorous acid."
Before getting into the final two types, you must be aware of the Greek prefixes used to name them. They only apply to the following two types of compounds, and are as follows:
(1) "mono-"
(2) "di-"
(3) "tri-"
(4) "tetra-"
(5) "penta-"
(6) "hexa-"
(7) "hepta-"
(8) "octa-"
(9) "nona-"
(10) "deca-"
You are probably already familiar with most of these, except the prefix for 4 is "tetra-" instead of "quad-" and the prefix for 7 is "hepta-" instead of "septa-".
Hydrates (Ionic Compound∙H2O)
Hydrates are probably the easiest to remember when naming. However, you should note that in their chemical formulas, the ionic compound and water compound are written with a dot in between rather than being combined like the other examples. If you see this a compound in this format, you would use the formula:

For example, the systematic name of washing soda (Na2SO4∙10H2O) would be sodium sulfate decahydrate.
Na2SO4 is an ionic compound containing sodium (Na) and sulfate (SO4) so it would be named "sodium sulfate". The H2O molecule has a 10 in front of it, meaning there are 10 water molecules in this hydrate, and thus, you would use the prefix "deca-" for 10 to add at the beginning of "hydrate" to get "decahydrate".
When you combine these, you would get the name "sodium sulfate decahydrate".
Molecular Compounds (Nonmetal-Nonmetal)
Molecular compounds are purely nonmetal and are formed by two elements or compounds with negative charges. These are also known as covalent bonds, usually formed by two or more gases. To name these, you would use the formula:

*note: you don't have to add the prefix "mono-" in the first anion if there is 1 atom of it present
For example, the systematic name of SF6 would be sulfur hexafluoride.
Since the first anion sulfur (S) doesn't have a subscript (a number that indicates how many atoms of the element there are), you wouldn't add any prefix, since "mono-" is not needed for the first element, so you would just leave it as "sulfur". Fluoride (F) has a subscript of 6 so you will take the root "fluor-" and add the prefix "hexa-" in the beginning and "-ide" at the end to get "hexafluoride". Therefore, you would write the final name as "sulfur hexafluoride".
And that concludes our list of rules for the different types of compounds! The most difficult part would be memorizing and understanding when to apply each rule according to the compound you are presented with. However, with a lot of practice using worksheets and sample problems, you'll definitely be able to master it soon! Thank you for reading!
References:
“Naming Compounds | Boundless Chemistry.” Lumen Learning, courses.lumenlearning.com/boundless-chemistry/chapter/naming-compounds. Accessed 10 Sept. 2020.




Comments