Structure of the Atom
- STEM To Go

- Oct 21, 2020
- 8 min read
Updated: Oct 22, 2020
All the matter around you, matter that you can and can't see, is made up of atoms. Atoms are the smallest particles of an element that still retain their chemical properties. The atom is the building block of all matter. Among the first to theorize about the its existence was the Greek philosopher Democritus who believed that atoms were indivisible and indestructible. More than 2000 years later, an English chemist named John Dalton used experimental methods to support Democritus' ideas through his own atomic theory. Dalton's atomic theory illustrated 5 main tenets;
All matter is composed of extremely small particles called atoms.
Atoms of one element are identical in size, mass, and other properties. Atoms of one element differ from atoms of different elements.
Atoms cannot be subdivided, created, or destroyed.
Atoms of different elements can combine in simple whole-number ratios to form chemical compounds.
In chemical reactions, atoms are combined, separated, or rearranged.
Although most of Dalton's atomic theory is accepted as true, it is now known that atoms are divisible and can be divided into three more fundamental subatomic particles. The three types of subatomic particles are electrons, protons, neutrons. The English physicist J.J. Thompson was the first to discover electrons, which are negatively charged particles. This was done through experiments involving an electric current being passed through low-pressure gases. The gases were sealed in glass tubes that had metal disks called electrodes fitted on either end. These electrodes were connected to a high voltage source of energy which caused one electrode, the cathode, to become negatively charged, and the other, the anode, to become positively charged. The result of this was a cathode ray, a glowing beam that traveled from the cathode to anode. This beam glowed because of the phosphors that were painted on the far end of the tube. When the cathode ray struck the phosphors, it would emit light and you would be able to see the cathode ray as a glowing beam. In order to test the properties of the particles that made up the cathode beam, Thompson added a plate with a slit for the beam to travel through so it could be more easily manipulated. He then used negatively charged and positively charged plating to attract the beam. He knew that opposite charges would attract and like charges would repel, so when the beam moved towards the positively charged plate, he could conclude that the beam was made up of negatively charged particles that were moving at a very high speed. He named these negatively charged particles corpuscles. They would later be named electrons.
To test his hypothesis, Thompson experimented to find the charge-to-mass ratio of a corpuscle. He found that the ratio was constant and that it did not depend on the type of gas in the tube or the type of metal in the electrodes. From this, he could conclude that an electron could be found in atoms of all elements. The physicist Robert A. Millikan experimented to find the amount of charge carried by an electron. He was able to calculate the mass of an electron and concluded that an electron carries 1 unit of negative charge and its mass is 1/1840 of the mass of a single hydrogen atom. This showed that an electron could only be a part of an atom.
Cathode rays are formed when atoms give off electrons. Since you don't get an electric shock every time you touch something, you know that atoms must be electrically charged. So something has to be in atoms that can cancel the electrons' negative charge. You know that electric charges can only be carried by a particle of matter. You also know that there are no fractions of electric charges and that, when negatively charged particles combine with an equal number of positively charged particles, a neutral particle is formed. With all of this, it only makes sense that there should be particles with one unit of positive charge in an atom. Evidence for these particles was found when Eugen Goldstein observed that there were rays traveling in the opposite direction of the cathode ray. He called them canal rays and found that they were made up of positively charged particles, which were named protons. The mass of a proton is 1840 times that of an electron.
With the discovery of subatomic particles, new questions arose. One of which was the question of how these particles fit together in the atom. At the time, it was widely believed by scientists that an atom was filled with positive protons and that the electrons were evenly distributed throughout this mass of positive energy. In fact, Thomson named his atomic model the "plum-pudding model" due to the fact that it was similar to the desert in which raisins were scattered throughout the dough. In American terms, it was like a chocolate-chip cookie.

Plum Pudding Model
However, this theory was soon challenged when a man named Ernest Rutherford and his colleagues decided to test it with an experiment. Using alpha particles, which are positively charged helium atoms that have lost both of their electrons, Rutherford designed an experiment in which he would allow a beam of these alpha particles to hit a thin sheet of gold foil in order to observe whether or not Thomson's theory would hold true. Since alpha particles are given off in many radioactive decay processes, Rutherford housed some radium in a lead box with a pinhole through which a tiny beam of alpha particles would be able to go through. This beam would hit the gold foil which was surrounded by a circlet of fluorescent detector on a screen. Based on the plum-pudding model, it was predicted that the alpha particles should be able to pass through the gold atoms which were supposedly filled with positively charged "soup" that would have too weak of a charge to effect the fast-moving alpha particles. But when the experiment was executed, the results were shocking. Instead of the predicted minimal deflection, some of the alpha particles bounced off the gold at wide angles. Some even bounced back in the direction of the source. Rutherford would later compare it to firing a bullet at a tissue and having it come back and hit you.

Rutherford's Gold Foil Experiment
These new findings led Rutherford to create his own atomic theory in which he proposed that the atom was filled with mostly empty space, which explained how most of the alpha particles could easily pass through the foil. This contradicted Thompson's theory in that the positive charge was not evenly distributed throughout the atom. Instead, Rutherford concluded that all the positive charge and most of the mass of the atom was located in a region that had enough positive charge to deflect the fast positively-charged alpha particles. He named this area the nucleus, the central core of an atom which consists of protons and neutrons. Due to this, Rutherford's model is also called the nuclear atom, in which electrons are distributed around the atom, and protons and neutrons are concentrated in the nucleus.
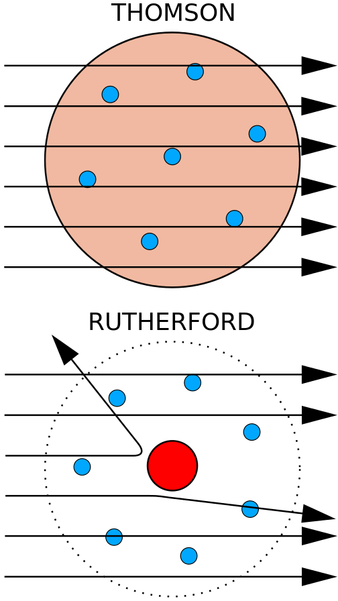
Thomson's model compared to Rutherford's model
Although this new theory seemed to cover all the bases, it could not explain the chemical properties of elements. If all atoms have protons and neutrons in the nucleus and electrons surrounding the nucleus, what makes a hydrogen atom different from a helium atom? The answer: protons. The atomic number of an atom is the number of protons in their nucleus. Hydrogen, with one proton, has the atomic number of 1, and helium, with two protons, has the atomic number of two. Since atoms are electrically neutral, you know that the number of electrons is equal to the number of protons in that atom. Rutherford's theory tells us that the mass of an atom is mostly located in its nucleus. Since the nucleus is made up of protons and neutrons, it follows that the mass number is the number of protons and neutrons in an atom. You can find the mass number by adding the number of protons and neutrons together. You can find the atomic number by subtracting the number of neutrons from the mass number.
But if the number of protons in an atom determines what element it is, what happens when the number of neutrons is different? For starters, this would change the mass number. Would they have different properties? Atoms that have the same number of protons but a different number of neutrons are called isotopes. Although they are the same element, their difference in neutrons makes them versions of each other. Since protons and electrons are the particles that determine the chemical behavior of atoms, isotopes have the same chemical properties. Hydrogen, for example, has three naturally occurring isotopes. The first, most common, is hydrogen-1 or just hydrogen which has no neutrons. The second is hydrogen-2 or deuterium which has 1 neutron. The third is hydrogen-3 or tritium which has 2 neutrons. When writing isotopes, there is a specific notation you must use. Standard nuclear notation tells us the chemical symbol, the mass number, and the atomic number of the isotope.
Here is a template for any isotope:

Standard Nuclear Notation
So the notation for hydrogen (protium), deuterium, and tritium would be as follows:

Hydrogen Isotope Notation
When we look at the mass of the subatomic particles in an atom, we see that electrons, protons, and neutrons have incredibly small masses that are written in scientific notation. The mass of one electron is 9.109 x 10-28 g, the mass of one proton is 1.673 x 10-24 g, and the mass of one neutron is 1.675 x 10-24 g. But writing these out often becomes tedious and inconvenient. So, instead, scientists use a reference isotope mass in order to describe other atoms relative to that isotope. Carbon-12 was chosen as the isotope and it was assigned a mass of 12 atomic mass units (amu). So the value of one amu is 1/12 of the carbon-12 atom's mass. In a carbon atom, there are 6 protons and 6 neutrons which account for nearly all the mass in the carbon-12 atom. Therefore, the mass of one proton or neutron is 1 amu. The mass of an electron is so small, it is considered negligible. Since different isotopes have different atomic masses, the atomic mass, which is the weighted average mass of the atoms in a naturally occurring element, is used to encompass all of the naturally occurring isotopes of a certain element. The mass is weighted because it includes the abundance of that isotope in nature. For example, the average atomic mass for all naturally occurring hydrogen isotopes is 1.0079 amu. But the masses for hydrogen-1, deuterium, and tritium are 1.0078 amu, 2.0141 amu, and 3.0160 amu respectively. The average atomic mass is much closer to the mass of hydrogen-1 than the other isotopes. This is because 99.985% of all naturally occurring hydrogen on earth is hydrogen-1 while 0.015% of hydrogen on earth is deuterium. For tritium, its percentage is so small, it is considered negligible. So the average atomic mass for hydrogen will be closest to hydrogen-1, which is the most abundant. The same goes for any other element's naturally occurring isotopes. To calculate the average atomic mass of an element, you multiply the mass of each stable naturally occurring isotope of that element by their respective natural abundance (decimal form) and add the products of each to get the weighted average mass.
Average Atomic Mass = (mass of isotope * natural abundance) + (mass of 2nd isotope * 2nd isotope's natural abundance) + ...
TL;DR (Too Long; Didn't Read)
Democritus believed that atoms were indivisible and indestructible.
Dalton's Theory: All matter is composed of atoms. Atoms of one element are identical in size, mass, and other properties. Atoms cannot be subdivided, created, or destroyed. Atoms of different elements can combine in simple whole-number ratios to form chemical compounds. In chemical reactions, atoms are combined, separated, or rearranged.
Three types of subatomic particles: proton (+ charge), neutron (neutral), electron (- charge)
Rutherford's nuclear atom: protons and neutrons are in the nucleus and make up most of the atom's mass. Electrons are distributed throughout the atom which is filled with mostly empty space.
The number of protons determines the element.
The atomic number is the number of protons in an element.
Protons + Neutrons = Mass Number
Isotopes are atoms that have the same number of protons but a different number of neutrons. They are different versions of an element and have a different mass but the same atomic number.
References:
“History of the Atom.” Science In Your Everyday Life, yostscience.weebly.com/history-of-the-atom.html.
Wilbraham, Antony C. Prentice Hall Chemistry. Prentice Hall, 2008.
Rutherford's Gold Foil Experiment Image Source: https://commons.wikimedia.org/wiki/File:Geiger-Marsden_experiment.svg
Rutherford's and Thomson's Atomic Model Image Source: https://commons.wikimedia.org/wiki/File:Gold_foil_experiment_conclusions.svg
Nuclear Notation Image Source: http://hyperphysics.phy-astr.gsu.edu/hbase/Nuclear/nucnot.html
Written by: Mahathi Somula




Comments