The Chemistry Behind Boba
- Stem To Go

- Jul 16, 2020
- 1 min read
Updated: Jul 17, 2020
Cold, sweet drinks are super refreshing during hot summers. One option that has been growing in popularity is boba tea, which you might have tried before! Keep reading to learn about how chemistry plays a role in the formation of the chewy tapioca balls you find in boba tea.
Tapioca balls are chewy, usually black spheres produced from tapioca, which is a starch extracted from the cassava root. Originating from Southeast Asian cuisine, they are commonly referred to as "boba", "pearls", or "bubbles".
Heat and water play a key role in the formation of tapioca balls. They promote the gelation of tapioca flour (and flour in general), which is a polymer molecule with amorphous and crystalline phases. The amorphous phase can be recognized by the randomly tangled polymers that resemble cooked noodles, while the crystalline phase looks more like organized, compressed stacks (see below).
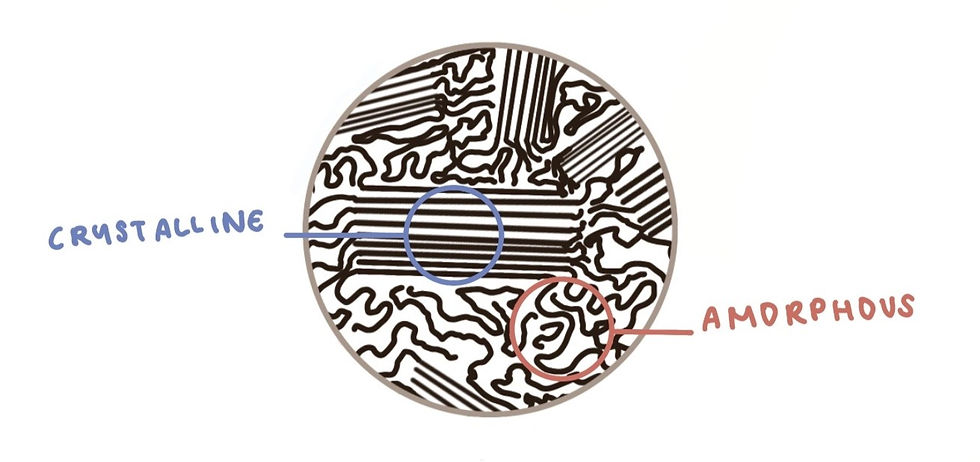
First, hot water molecules enter the amorphous region and loosen the polymer structure.

As more water enters the polymer structure, the heat from the water loosens the closely packed crystalline regions. This uncoiling action results in the formation of long chain polymers that create hydrogen bonds with each other. This initiates gelation, forming tapioca balls that are softer to chew and easier to digest.

I hope you enjoyed learning about how boba balls are formed!
Written by: Ashlee Liu
References:
Zhuojun, View All Posts By Huang. “Breaking Boba – the Chemistry of Bubble Tea (Pt. 1).”The Big Nano, 29 Jan. 2019, itsamaterialsworld.wordpress.com/2018/12/26/breaking-boba-the-chemistry-of-bubble-tea-pt-1.




Comments