Classifying Matter
- Stem to go

- Sep 5, 2020
- 2 min read
Atoms - the smallest units of an element that maintain the chemical identity of that element- are the smallest stable units of matter.
And matter, anything that has mass and volume, describes almost everything in our life. But how do we classify matter, or break them into groups to bring better understanding? Read this article to find out!
Definition
Let’s start with the elements. An element is a pure substance that cannot be broken down into simpler, stable substances. It is made of one type of atom. Elements are listed on the periodic table.

Compounds are a kind of substance that can be broken down into simpler stable substances. They are made from the atoms of two or more elements that are chemically bonded. An example would be water molecules, which consist of two hydrogens and one oxygen (H2O)
Molecules are two or more atoms chemically bonded together. All compounds are molecules, but not all molecules are compounds. Why? Compounds contain two or more different atoms, while for molecules, the atoms can be the same.
Furthermore, not only do we classify matters by the chemical bonds, but also physical bonds. Mixtures, which can be further classified as a mixture of elements, a mixture of compounds, and a mixture of elements and compounds, is when different types of atoms are together but not connecting to each other through chemical bonds. An example would be the air, a mixture containing oxygen, nitrogen, and more.
Practice
Now you know the definition of the terms, but what exactly do elements, compounds, and mixtures look like?
If a little sphere represents an atom, then elements look like this:

And if the black and white spheres represent different types of atoms, compounds look like this:

Molecules, which can be either elements or compounds, look like this:

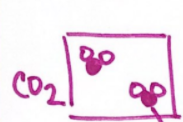
A mixture of elements, where there are both black and white spheres but they are not interconnected:
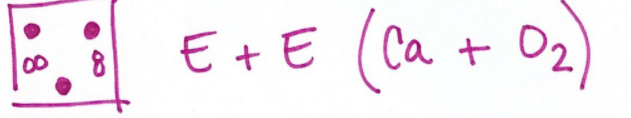
A mixture of compounds, where black and white spheres joined differently first, then together they are blended.
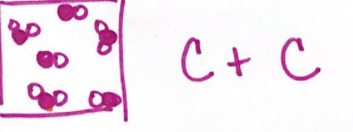
A mixture of elements and compounds would look like this:
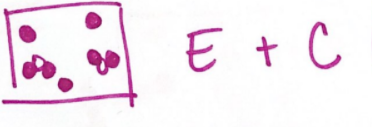
Here is a practice sheet that you may want to check out. The key is attached after the worksheet.
Each circle represents an atom and each different color represents a different kind of atom. If two atoms are touching then they are bonded together. Classify each of the pictures below. (Possible answers: element, compound, mixture of elements, mixture of compounds, mixture of elements and compounds)
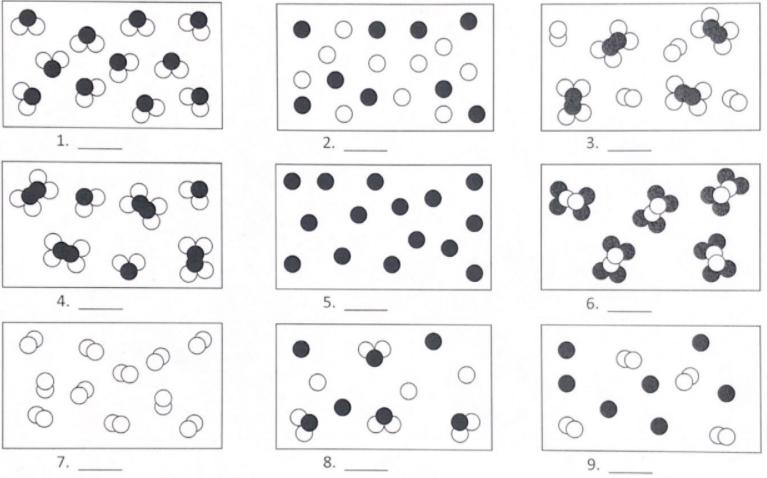
(Answers:
1. compound; 2. mixture of elements; 3. mixture of elements and compounds; 4. mixture of compounds; 5. element; 6. compound; 7. element; 8. mixture of elements and compounds; 9. mixture of elements
Everything might seem confusing and doesn’t make sense at first, but practice makes perfect. When dealing with things that are especially definition based, there’s nothing to worry about but keep practicing!
Written by: Benetta Wang



Comments